Solution Detail:-
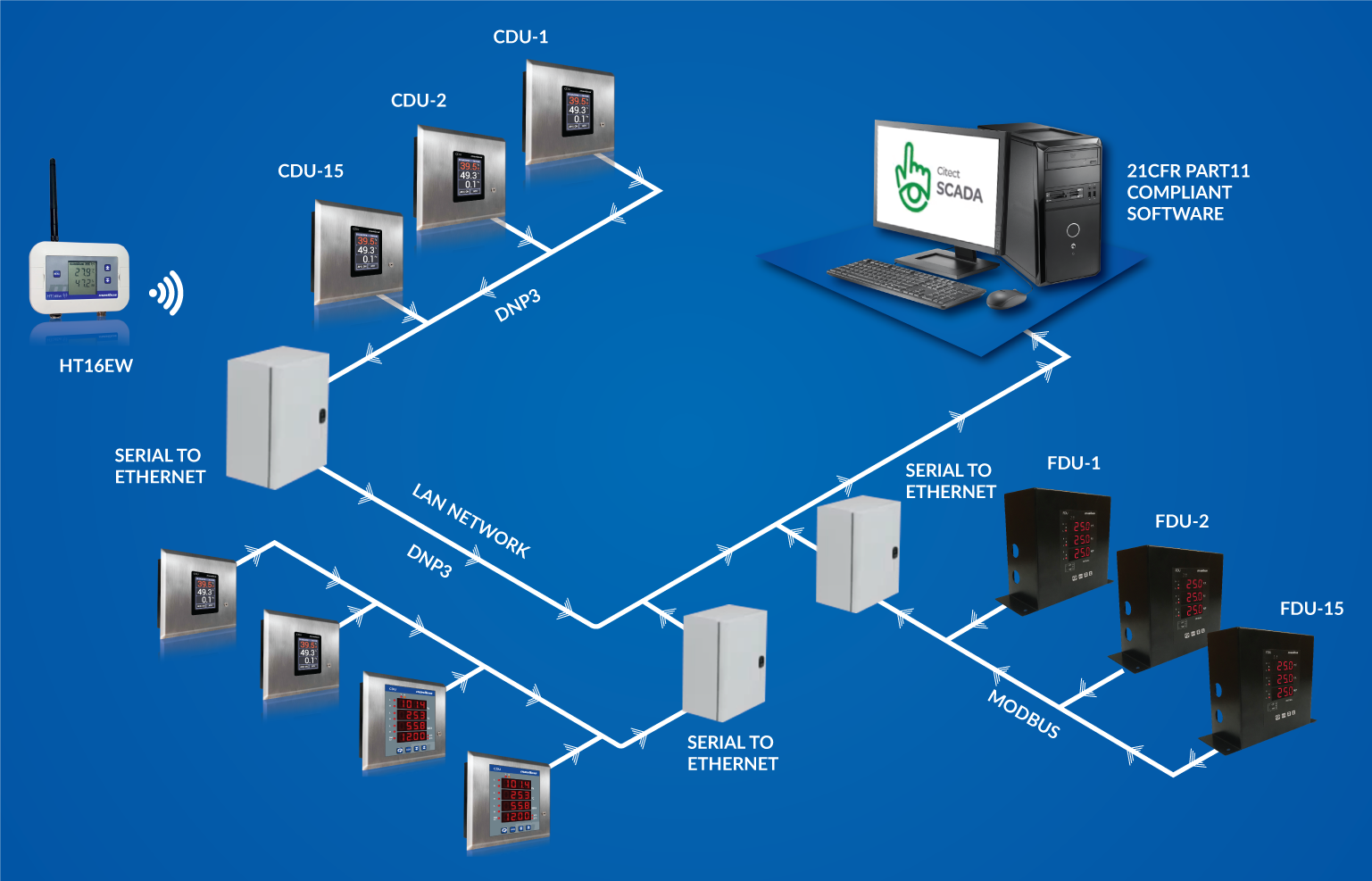
Export oriented Pharma plants and USFDA approved Pharma plants require 21CFR Part11 compliant Environment Monitoring System to comply with FDA requirements. It is very crucial for Pharma formulation and API facility to maintain and monitor desired temperature, humidity and differential pressure in different pharma plant areas like clean rooms, warehouses, API reactors etc. Masibus provides complete state of the art architecture for Pharma environment monitoring system helping the plant to comply with FDA norms and keeping the execution seamless as we provide hardware and software as a package. Masibus EMS consists of LED, LCD clean room display units, flameproof variants, wireless humidity and temperature monitoring system supported by DNP protocol which assures data availability at hardware level. Our solution is scalable and flexible to cater to future expansion and ensures reliability and availability by redundancy at software and hardware server level.